Abstract
Introduction Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma that includes 3 subtypes: extranodal marginal zone lymphoma (EMZL) of the mucosa-associated lymphatic tissue (MALT), splenic MZL (SMZL), and nodal MZL (NMZL). Although flow cytometry can detect circulating lymphoma cells in many cases of MZL, outside of case reports and small case series, the relevance of this circulating lymphoma (CL) in patients with MZL at diagnosis is largely unknown. Hence, we sought to evaluate the impact of CL at diagnosis on outcomes in patients with MZL.
Methods This multicenter, retrospective cohort study of MZL patients treated at 3 US medical centers included patients who were ≥18 years old, diagnosed with MZL from 2010-2020, and had peripheral blood flow cytometry performed at diagnosis. Patients who never received therapy for MZL were excluded. Patients were classified according to positive or negative flow cytometry results as CL+ and CL-, respectively. The primary endpoint was progression-free survival (PFS) defined as the time from the start of first-line therapy until lymphoma relapse/progression or death from any cause, censoring at the last clinical assessment. Secondary endpoints were overall survival (OS, from the start of first-line therapy until death or last follow-up) and time from diagnosis to systemic therapy in CL+ and CL- groups.
Results Among 294 eligible patients with newly diagnosed MZL, 75 (25%) had detectable CL at diagnosis. The CL+ group had a higher median age (66 vs 63 years), advanced-stage disease (100% vs 53%), bone marrow involvement (91% vs 35%), elevated LDH (49% vs 22%), lower albumin (24% vs 13%), and less often received R-chemotherapy (39% vs 41%) compared to the CL- group. SMZL constituted the predominant subtype (52%, n=39) in the CL+ group with NMZL and EMZL constituting 24% each (n=18).
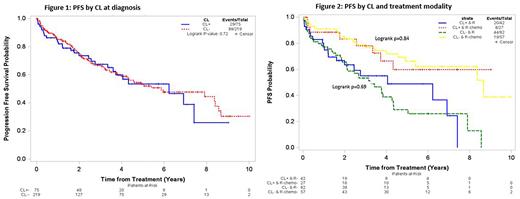
The median PFS was not significantly different between the CL+ (6.2 years, 95%CI=3.6-NR) and CL- (5.9 years, 95%CI=4.3-8.4), p=0.73 (Figure 1) groups. Similar results were noted across the MZL subtypes with no significant difference in median PFS in EMZL (p=0.51), NMZL (p=0.51), or SMZL (p=0.38) according to the CL status. The median PFS was not significantly different between CL+ and CL- groups among patients treated with rituximab monotherapy (median PFS 4.1 vs 3.2 years, respectively, p=0.69) or immunochemotherapy (median PFS NR vs 8.7 years, respectively, p=0.84, Figure 2).
After adjusting for factors associated with PFS in multivariate cox regression (age, B symptoms, albumin level, LDH>ULN, and type of first-line treatment regimen), there was no difference in PFS between CL+ and CL- groups (HR=0.73, 95%CI=0.45-1.20, p=0.22).
We did not observe a significant difference in OS between the CL+ and CL- groups with a 5-year rate of OS being 89% vs 82% in the CL+ and CL- groups (p=0.31). Furthermore, the time from diagnosis to systemic therapy initiation did not significantly differ between the CL+ and CL- groups with % of patients who started treatment at 1, 3, and 5 years from diagnosis being 83% vs 92%, 95% vs 96%, and 97% vs 99%, respectively (p=0.42).
Conclusions This is the first study to compare the outcomes of patients with CL+ and CL- groups in newly diagnosed MZL. In this largest study to date evaluating the prognostic relevance of CL in patients with MZL, we found that CL at diagnosis was not associated with shorter PFS, OS, or time from diagnosis to first-line therapy. Further studies should evaluate whether the presence of CL is associated with specific molecular or other clinically relevant markers that were not available in our patient population.
Disclosures
Grover:ADC: Other: Advisory Board; Kite: Other: Advisory Board; Novartis: Consultancy; Tessa Therapeutics: Consultancy; Genentech: Research Funding. Christian:Acerta: Research Funding; Celgene/Bristol-Myers Squibb: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Millennium: Research Funding; Triphase: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Olszewski:TG Therapeutics: Consultancy, Research Funding; Schrodinger: Consultancy; Acrotech Biopharma: Research Funding; Celldex: Research Funding; Adaptive Biotechnologies: Research Funding; Precision Bio: Research Funding; Genmab: Consultancy, Research Funding; Genentech: Research Funding. Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal